-
Magnesium-Adenosine Diphosphate binding sites in wild-type creatine kinase and in mutants:role of aromatic residues probed by Raman and Infrared spectroscopies
H. Hagemann, O. Marcillat, R. Buchet and C. Vial
Biochemistry, 39 (31) (2000), p9251-9256


DOI:10.1021/bi000009d | unige:3605 | Abstract | Article HTML | Article PDF
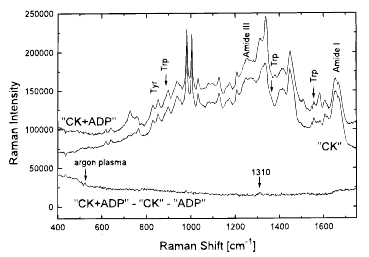
Two distinct methods were used to investigate the role of Trp residues during Mg-ADP binding to cytosolic creatine kinase (CK) from rabbit muscle: (1) Raman spectroscopy, which is very sensitive to the environment of aromatic side-chain residues, and (2) reaction-induced infrared difference spectroscopy (RIDS) and photolabile substrate (ADP[Et(PhNO2)]), combined with site-directed mutagenesis on the four Trp residues of CK. Our Raman results indicated that the environment of Trp and of Tyr were not affected during Mg-ADP binding to CK. Analysis of RIDS of wild-type CK, inactive W227Y, and active W210,217,272Y mutants suggested that Trp227 was not involved in the stacking interactions. Results are consistent with Trp227 being essential to prevent water molecules from entering in the active site [as suggested by Gross, M., Furter-Graves, E. M., Wallimann, T., Eppenberger, H. M., and Furter, R. (1994) Protein Sci. 3, 1058−1068] and that another Trp could in addition help to steer the nucleotide in the binding site, although it is not essential for the activity of CK. Raman and infrared spectra indicated that Mg-ADP binding does not involve large secondary structure changes. Only 3−4 residues absorbing in the amide I region are directly implicated in the Mg-ADP binding (corresponding to secondary structure changes less than 1%), suggesting that movement of protein domains due to Mg-nucleotide binding do not promote large secondary structure changes.